Vocabulary Preview
- Counterintuitive: Contrary to intuition or to common-sense expectation.
- Ubiquitous: Present, appearing, or found everywhere.
- Mundane: Lacking interest or excitement; dull; of this earthly world rather than a heavenly or spiritual one.
- Anomaly: Something that deviates from what is standard, normal, or expected.
- Lattice: A structure consisting of strips of wood or metal crossed and fastened together with square or diamond-shaped spaces left between, used metaphorically for crystal structures.
- Insulate: To protect (something) by interposing material that prevents the loss of heat or the intrusion of sound.
- Serendipitous: Occurring or discovered by chance in a happy or beneficial way.
- Cohesion: The action or fact of forming a united whole; in physics, the sticking together of particles of the same substance.
- Fluctuate: To rise and fall irregularly in number or amount.
- Profound: (of a state, quality, or emotion) very great or intense; having or showing great knowledge or insight.
Listen
Or Read
Why Does Ice Float on Water?
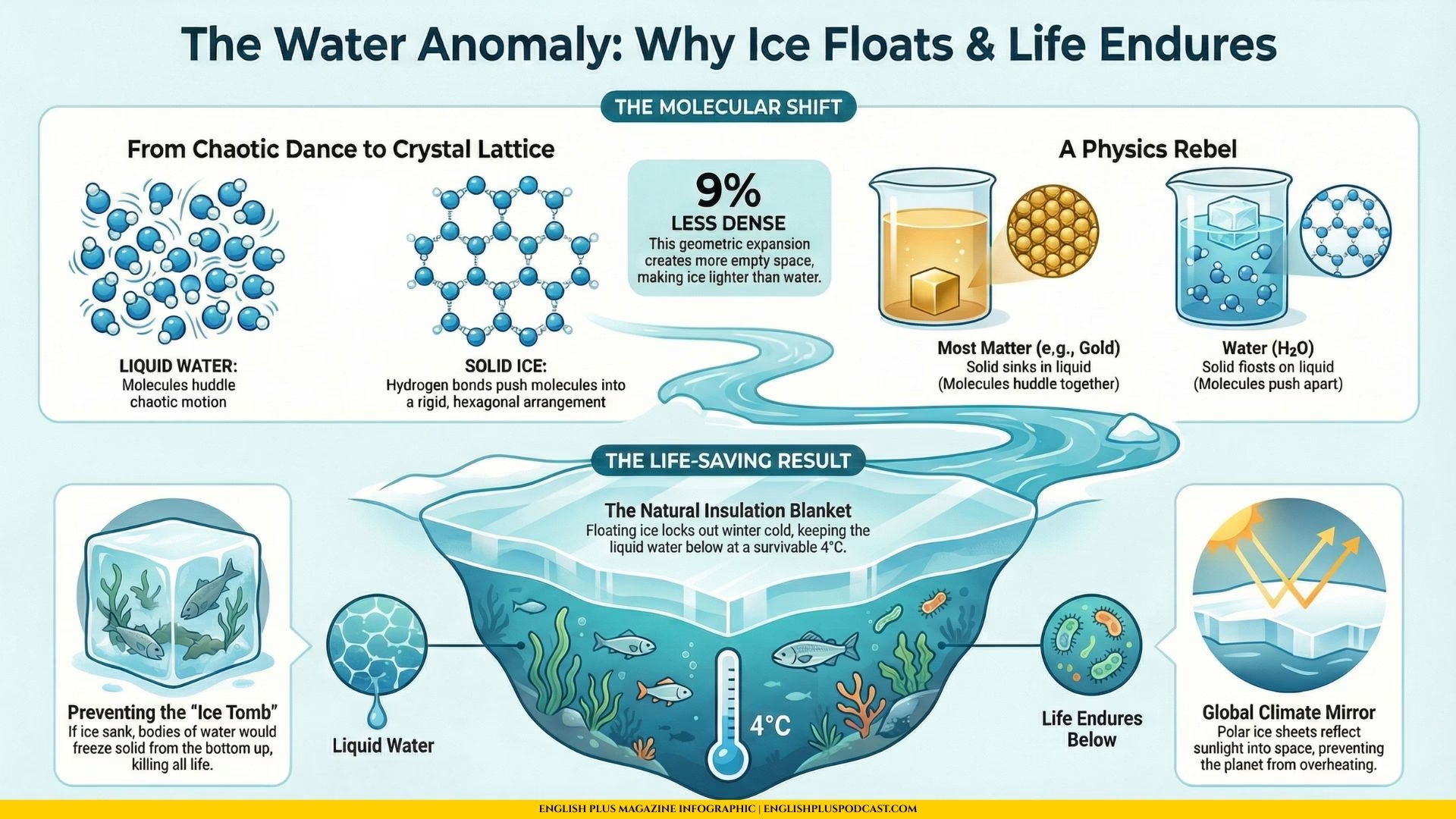
We are so used to seeing ice cubes clinking against the glass rim that we rarely stop to consider just how bizarre this behavior actually is. In the vast majority of the universe, when a liquid cools down and turns into a solid, the molecules huddle closer together, packing themselves tightly to conserve heat and energy. This usually makes the solid form heavier, or denser, than the liquid form. If you melted a bar of gold and then tossed a solid nugget of gold into it, that nugget would sink straight to the bottom. But water? Water is the rebel of the periodic table. It is a counterintuitive substance that decides to break the rules just when things get chilly. This strange behavior is known as the water anomaly, and while it might seem like a trivial bit of trivia you’d find on a Snapple cap, it is actually the reason life on Earth exists at all.
To understand this, we have to shrink ourselves down to the size of an atom and look at what is happening on a mundane level.
A water molecule, good old H2O, is shaped a bit like Mickey Mouse’s head, with one big oxygen atom and two smaller hydrogen ears. These molecules are incredibly sticky; they love to hold hands with each other thanks to something called hydrogen bonds. In liquid water, these molecules are like a chaotic dance floor at a wedding—everyone is moving around, bumping into each other, holding hands for a second, and then letting go to grab someone else. There is a lot of energy, and they are packed relatively close together.
But as the temperature drops and water begins to freeze, the music slows down. The molecules stop wiggling so much and decide to settle into a permanent arrangement. Here is where the magic happens. Instead of huddling tight like penguins in a storm, the hydrogen bonds push the water molecules apart to form a rigid, hexagonal crystal structure.
Think of it like a social distancing protocol for atoms. This structure is called a crystal lattice. Because the molecules are holding each other at arm’s length to maintain this beautiful geometric shape, there is actually more empty space between them in ice than there is in liquid water. This extra space means that ice is about 9% less dense than liquid water. And in the world of physics, if you are less dense than the liquid you are sitting in, you float. It is a simple rule with profound consequences.
Now, you might be thinking, “Okay, so my drink stays cold at the top. Who cares?” Well, the fish care. A lot. Imagine if water behaved like a “normal” substance. If ice were denser than water, it would sink to the bottom of lakes and oceans. In winter, the top layer of water would freeze, sink, and pile up on the riverbed. Then the next layer would freeze and sink on top of it. Eventually, the entire body of water would turn into a solid block of ice from the bottom up. This would freeze every fish, plant, and microorganism in a tomb of solid ice. Summer might melt the top few inches, but the depths would likely remain frozen forever. Our planet would look less like a blue marble and more like a white, lifeless snowball.
Instead, because ice floats, it forms a protective barrier on the surface. This layer of ice acts like a blanket to insulate the water below. It locks out the brutal cold of the winter air, keeping the liquid water underneath at a survivable temperature—usually around 4 degrees Celsius, where water is actually at its densest. This allows the aquatic ecosystem to survive the winter in a dark but safe liquid haven. It is a serendipitous quirk of chemistry that turns a potentially lethal season into a cozy slumber party for marine life.
This property also affects our climate on a global scale. The vast sheets of ice at the poles act like giant mirrors, reflecting sunlight back into space and helping to regulate the Earth’s temperature. If that ice sank, the dark ocean water would absorb all that heat, and our planet would be a much hotter, more chaotic place. The cohesion of the water molecules, their ability to stick together and then push apart when frozen, drives weather patterns and ocean currents that stabilize our entire world. It is staggering to think that the ubiquitous substance coming out of your kitchen tap holds such power in its molecular geometry.
So, the next time you see an iceberg in a documentary, or just an ice cube in your lemonade, don’t just look at it as a cooling agent. Look at it as a tiny life raft. It represents a defiance of the standard laws of physics, a lucky break in the grand design of the universe that allows biology to flourish in a universe that is mostly hostile. It’s a reminder that sometimes, being different, being the anomaly, is exactly what is needed to survive. We often take the stability of our environment for granted, assuming that the way things are is the only way they could be. But nature is fragile, balanced on these tiny, specific details that fluctuate only slightly.
It makes you wonder, doesn’t it? If water played by the rules of every other liquid, we wouldn’t be here to talk about it. It’s a humbling thought to realize our existence hinges on the angle of a hydrogen bond. So, here is a question to leave you with: What other “ordinary” things in your daily life do you think might be hiding extraordinary secrets that keep your world turning? I’d love to hear your theories in the comments below!
Word Power
Let’s take a closer look at the vocabulary we just used to decode the mystery of the floating ice. These words are fantastic tools for describing complex ideas in a way that makes you sound both smart and relatable.
First up, we have Counterintuitive. This is a great word for when something happens that is the opposite of what you expect. You expect a solid rock to sink, right? So when a solid rock of ice floats, that is counterintuitive. You might use this in real life when talking about advice, like, “It seems counterintuitive, but to get more work done, you actually need to take more breaks.”
Then we used Ubiquitous. This just means “everywhere.” Water is ubiquitous. Smartphones are ubiquitous. It’s a fancy way of saying something is very common. If you go to a city and see a Starbucks on every corner, you could say, “Coffee shops are ubiquitous in this neighborhood.”
We described the molecular level as Mundane. Usually, mundane means boring or ordinary, like doing laundry or paying taxes. But in science, it can refer to the earthly, physical reality of things. We used it to contrast the magical result (life exists!) with the boring cause (atoms moving around). You can use it to complain about your day: “I’m tired of these mundane tasks; I want adventure!”
Anomaly is a key word here. It means something that doesn’t fit the pattern. Water is an anomaly because it expands when it freezes. In data analysis or business, if you see a sudden spike in sales for no reason, that’s an anomaly. It’s a break in the rule.
We talked about the crystal Lattice. A lattice is usually a crisscross pattern of wood you see on a garden fence. In chemistry, it describes the structured 3D grid of atoms. It paints a clear picture of structure and order.
Insulate is a practical word. It means to protect something from losing heat or sound. You wear a coat to insulate your body. We said ice insulates the water below. You can also use it metaphorically: “He tried to insulate his children from the harsh news.”
Serendipitous is a beautiful word. It refers to a happy accident. Finding a twenty-dollar bill in your old jeans is serendipitous. We used it to say that water’s weird behavior is a lucky break for life on Earth.
Cohesion refers to sticking together. A team has good cohesion if they work well together. Water molecules have high cohesion, which is why water forms droplets instead of spreading out flat instantly.
Fluctuate means to go up and down unpredictable. Stock prices fluctuate. The temperature fluctuates in the spring. It implies instability or change.
Finally, Profound. This means deep, intense, or having great significance. A simple rule having a massive impact is profound. You might have a profound realization about your career, or read a book that had a profound effect on you.
Speaking Tips & Challenge
Now, how can you use these words without sounding like you swallowed a dictionary? The trick is to use them when you want to add weight or precision to your story.
- Tip 1: Use Counterintuitive when you are explaining a surprising fact to a friend. “I know it sounds counterintuitive, but putting salt on watermelon makes it sweeter.”
- Tip 2: Use Mundane to bond over shared boredom. “Let’s skip the mundane stuff and talk about your trip!”
- Tip 3: Use Anomaly when pointing out something weird. “That warm day in January was a total weather anomaly.”
Your Speaking Challenge:
I want you to observe your environment today. Find one thing that is ubiquitous (everywhere) and one thing that is an anomaly (weird or out of place).
Describe them to yourself or a friend using those words.
Example: “Construction noise is ubiquitous in this city, but that silent park in the center is a beautiful anomaly.”
Go explore your world!








0 Comments